Dr. Marraffa
Hello. I am Jeanna Marraffa, Pharm.D., Diplomate of the American Board of Applied Toxicology, assistant clinical director of the Upstate New York Poison Center of Upstate Medical University, and associate professor in the Department of Emergency Medicine at Upstate Medical University in Syracuse, New York. The goals of this case study are to discuss the issue of loperamide abuse and to present a patient who experienced cardiotoxicity and multiple episodes of torsades de pointe after taking supratherapeutic doses of loperamide to self-manage opioid withdrawal.
Patient Case Study
Name: Anna M.
Sex: Female
Age: 43 years
Clinical Findings
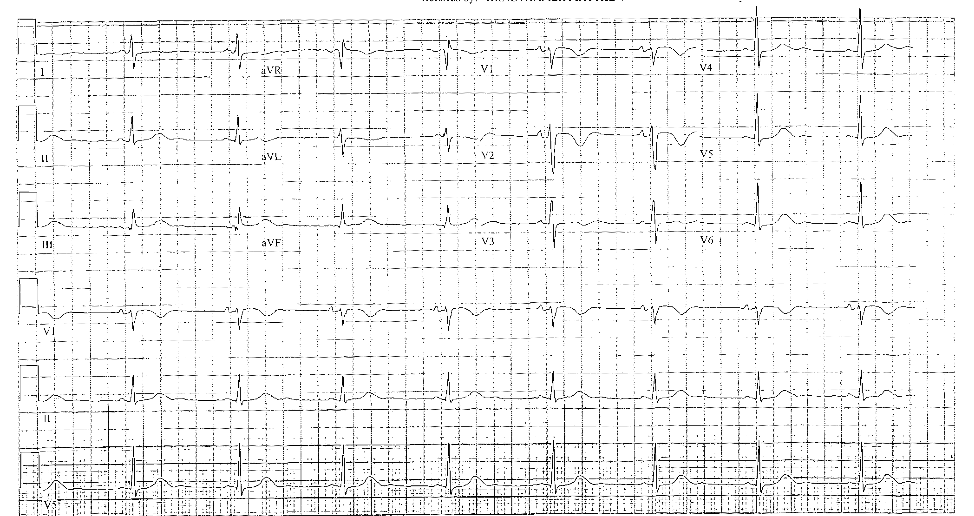
A 43-year-old female presented to the emergency department (ED) with syncope. While in the ED, she experienced multiple episodes of torsades de pointe (TdP) and ventricular arrhythmias. Intravenous lidocaine, amiodarone, sodium bicarbonate, magnesium, and fatty acid emulsion therapy were all given. She required repeated cardioversion, with at least 15 shocks. Control was ultimately achieved by transvenous pacemaker insertion with overdrive pacing. She required overdrive pacing for three days.
She reported taking 144 tablets of loperamide (each 2 mg) daily, totaling 288 mg, to manage symptoms of opioid withdrawal.
Patient History:
Opioid use disorder
No prescription medications
Understanding Loperamide Abuse and the Risk of Severe Cardiotoxicity
Poison centers provide vital services and information related to poison exposure and educate both the public and healthcare professionals on topics in toxicology, including emerging drug threats. Through the National Poison Data System (NPDS), real-time toxicosurveillance is performed, making it possible to identify trends. NPDS data show that loperamide misuse and abuse have increased in incidence over the past several years in the United States. With this increase, unexpected cardiotoxicity has emerged. The increased number of reports of severe cardiotoxicity resulted in a Food and Drug Administration Drug Safety Communication warning clinicians of the issue in 2016. In early 2018, the FDA suggested loperamide packaging changes to encourage safe use and is working with manufacturers on this initiative.
Loperamide is a readily available, over-the-counter (OTC) antidiarrheal agent that is safe and effective when used according to label directions. It inhibits intestinal peristalsis through mu-opioid receptor agonism, calcium channel blockade, calmodulin inhibition, and decreasing paracellular permeability. Its low oral systemic bioavailability and minimal central nervous system penetration, which are due to its extrusion from the CNS by P-glycoprotein efflux pump, limit abuse potential when taken at therapeutic doses.1 In fact, from the 1980s — when it was first made available as an OTC product — through 2007, it was classified as “free of abuse potential.”1 However, at supratherapeutic doses or when combined with xenobiotics that inhibit P-glycoprotein, loperamide can cross the blood-brain barrier and exert opioid effects.
The number of loperamide abuse cases is small but increasing, particularly over the last decade. Daniulaityte et al.2 first reported the extramedical use of loperamide among illicit opioid users in 2013. Publications in medical literature since 2013 describe this trend of increasing misuse and abuse of loperamide. Borron et al.3 performed a retrospective review of the NPDS database from 2009 through 2015 and demonstrated a significant increase in loperamide exposures reported to poison centers. The exact incidence of loperamide abuse is difficult to measure due to low case counts and frequent involvement of polypharmacy. Nevertheless, healthcare providers’ awareness of this type of abuse is important to ensure appropriate treatment.
Users report excessive doses of loperamide, with some reporting doses of more than 400 mg per day with or without xenobiotics that are P-glycoprotein inhibitors, such as quinine, cimetidine, and proton pump inhibitors.
Prior to 2014, the risk of cardiotoxicity secondary to loperamide use was not recognized, largely because this cardiotoxicity is not seen with therapeutic doses. In 2014, my colleagues and I4 published the first case series of five patients with loperamide abuse and severe cardiac conduction disturbances. Since that time, there have been additional case reports and case series published in the medical literature describing cardiotoxicity related to loperamide abuse and misuse. The reports include cases of QRS widening, QT prolongation, ventricular arrhythmias, and death after loperamide abuse.5,6,7 Swank et al.8 recently reviewed all of the cases reported to FDA MedWatch, the adverse event reporting system used in postmarketing drug surveillance. Reporting to MedWatch is voluntary for healthcare professionals and consumers; however, it is mandatory for drug manufacturers. The authors sought to characterize the postmarketing reports of cardiotoxicity associated with loperamide use from December 1976 through December 2015. There were 48 cases of serious adverse cardiac events, with 10 deaths. The median daily dose of loperamide in these cases was 250 mg (range: 70–1600 mg).
At very high concentrations, loperamide and its metabolite, N-desmethyl-loperamide, have been shown to block both the human ether-a-go-go-related gene potassium channels and sodium channels.9,10,11 This further strengthens the association described in the published case reports and case series.
Acute overdoses of loperamide have not resulted in cardiotoxicity. Data have shown that loperamide is a cardiac toxin and impairs cardiac conduction, but a clear dose-response relationship has yet to be described. Previously described as an inactive metabolite, N-desmethyl-loperamide likely plays a significant role in the cardiotoxicity seen, and there appears to be an increased risk of cardiotoxicity in patients who are chronically using excessively high doses of loperamide.
Treatment
The management of loperamide toxicity is largely supportive. However, early recognition of exposure to loperamide is essential. This may be difficult, because loperamide is not included in a standard toxicology screen.
If the patient presents with opioid overdose and respiratory depression, naloxone should be administered. The lowest effective dose of naloxone should be used to minimize withdrawal symptoms.
In patients with acute overdose, loperamide does adsorb to activated charcoal and should be considered as long as there are no contraindications. There is no role of activated charcoal in the chronic overdose setting.
In all cases of suspected loperamide overdose, an electrocardiogram (ECG) should be obtained, especially for patients with syncope or with a known history of loperamide abuse. While QT prolongation is more likely to occur, there have been cases of QRS complex widening.
In patients with loperamide cardiotoxicity, standard advanced cardiovascular life support (ACLS) therapy should be initiated. These measures include cardioversion or defibrillation, intravenous magnesium for TdP, sodium bicarbonate for wide QRS complex, and transvenous pacing or isoproterenol for TdP. Several of the case reports describe the need for repeated shocks.
If you suspect loperamide abuse or overdose, consider a test for the patient’s loperamide parent and metabolite levels once the patient is stable. It is important to obtain loperamide blood levels, document the time from ingestion, and report this information to your local Poison Control Center.
A reasonable treatment approach includes the following:
- Obtain ECG and evaluate QRS/QT intervals.
- Administer sodium bicarbonate 1–2 mEq/kg IV bolus for wide QRS complex.
- Replace K and Ca to normal levels if QTc is prolonged > 500 msec.
- May give 2 g magnesium IV if QTc is prolonged > 500 msec.
- If TdP occurs:
- Perform standard ACLS for TdP (electricity and magnesium).
- Provide overdrive pacing to maintain a heart rate > 100 bpm or isoproterenol to maintain a heart rate > 100 bp.
- If resuscitation efforts are failing, may give 100 ml IV bolus of 20 percent fatty acid emulsion, though it is unclear whether this is helpful.
The duration of cardiotoxicity after chronic loperamide abuse is largely unknown. The case reports describe prolonged cardiotoxicity of several days, suggesting that loperamide and N-desmethyl-loperamide have a prolonged half-life at supratherapeutic concentrations. Because of this, patients may require treatment for several days.
Once the patient has been stabilized, it is imperative to address management of the underlying opioid use disorder.
Key Considerations
When used at therapeutic doses and according to label directions, loperamide is safe and effective to treat diarrhea.
Opioid use disorder is a public health crisis in the U.S. There were more than 42,000 deaths in 2016 involving opioids as reported by the Centers for Disease Control (CDC). Preliminary data from the CDC show that there were an estimated 72,000 deaths in 2017 due to drug overdoses, with a large percentage of these overdoses involving opioids.
The number of loperamide misuse and abuse cases is small but seems to be increasing. This could be due to the relative ease of access, relatively low cost, and under-recognition of its abuse potential.
Unexplained syncope or ventricular arrhythmias should prompt clinicians to consider loperamide in the differential diagnosis, especially when there is a history of opioid use disorder.
Patients with loperamide cardiotoxicity should be managed supportively and in consultation with a regional poison control center or toxicologist.
Cases of suspected cardiotoxicity should be reported to the regional poison control center and to FDA MedWatch.
Educating healthcare professionals, especially those in the treatment and prevention community, about the risk of loperamide cardiotoxicity is crucial.
Further study is necessary to describe the mechanism of cardiotoxicity and to identify a dose-response curve to risk-assess patients.
References
- Baker DE. Loperamide: A pharmacological review. Rev Gastroenterol Disord. 2007;7(SUPPL. 3):S11-S18.
- Bishop-Freeman SC, Feaster MS, Beal J, et al. Loperamide-Related Deaths in North Carolina. J Anal Toxicol. 2016;40(8):677-686. doi:10.1093/jat/bkw069.
- Borron SW, Watts SH, Tull J, Baeza S, Diebold S, Barrow A. Intentional Misuse and Abuse of Loperamide: A New Look at a Drug with “Low Abuse Potential.” J Emerg Med. 2017;53(1):73-84. doi:10.1016/j.jemermed.2017.03.018.
- Daniulaityte R, Carlson R, Falck R, et al. “I just wanted to tell you that loperamide WILL WORK”: A web-based study of extra-medical use of loperamide. Drug Alcohol Depend. 2013;130(1):241-244. doi:10.1016/j.drugalcdep.2012.11.003.
- Eggleston W, Clark KH, Marraffa JM. Loperamide Abuse Associated With Cardiac Dysrhythmia and Death. Ann Emerg Med. 2017;69(1):83-86. doi:10.1016/j.annemergmed.2016.03.047.
- Kang J, Compton DR, Vaz RJ, Rampe D. Proarrhythmic mechanisms of the common anti-diarrheal medication loperamide: revelations from the opioid abuse epidemic. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(10):1133-1137. doi:10.1007/s00210-016-1286-7.
- Marraffa JM, Holland MG, Sullivan RW, et al. Cardiac conduction disturbance after loperamide abuse. Clin Toxicol. 2014;52(9):952-957. doi:10.3109/15563650.2014.969371.
- Sheng J, Tran PN, Li Z, et al. Characterization of loperamide-mediated block of hERG channels at physiological temperature and its proarrhythmia propensity. J Pharmacol Toxicol Methods. 2017;88:109-122. doi:10.1016/j.vascn.2017.08.006.
- Swank KA, Wu E, Kortepeter C, McAninch J, Levin RL. Adverse event detection using the FDA post-marketing drug safety surveillance system: Cardiotoxicity associated with loperamide abuse and misuse. J Am Pharm Assoc. 2017;57(2, Supplement):S63-S67. doi:10.1016/j.japh.2016.11.011.
- Vaz RJ, Kang J, Luo Y, Rampe D. Molecular determinants of loperamide and N-desmethyl loperamide binding in the hERG cardiac K+ channel. Bioorg Med Chem Lett. 2018;28(3):446-451. doi:10.1016/j.bmcl.2017.12.020.
- Wu PE, Juurlink DN. Clinical Review: Loperamide Toxicity. Ann Emerg Med. 2017;70(2):245-252. doi:10.1016/j.annemergmed.2017.04.008.